
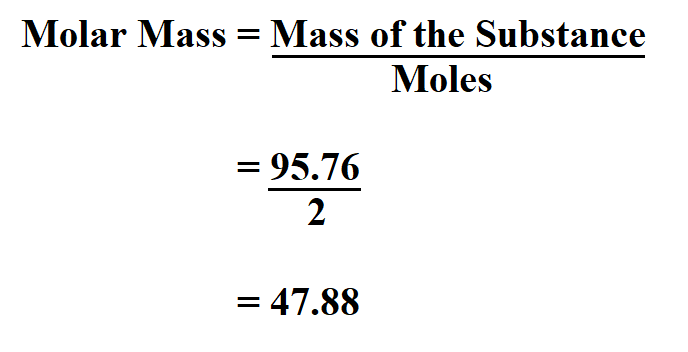
Amadeo Avogadro first proposed that at a given temperature and pressure, the volume of a gas is proportional to the number of atoms or molecules, and has nothing to do with the type of gas.Īlthough he did not define the exact ratio, he owes the idea to him. Avogadro’s Number:Īmedeo Avogadro is considered to be the number of units (usually molecules or atoms) in a substance that is proportional to its physical mass. The molar mass of the NaOH compound is 40 g/mol. Now, molecular mass calculator add all masses of substances together:ġ6 g / mol + 23 g / mol + 1 g / mol = 16 g / mol + 40 g / mol 23 g / mol + 1 g / mol = 40 g / mole In the NaOH compound, the molar mass of Na alone is 23 g/mol, the molar mass of O is 16 g/mol, and the molar mass of H is 1 g/mol. Therefore, the molar mass of Na2CO3 is 106 g/mol. If molecular formula calculator add up the total value, which is 12 + 46 + 48 = 106
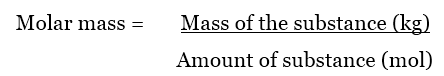
Since sodium carbonate contains one carbon atom, two sodium atoms, and three oxygen atoms, the molecular weight is What is the molar mass of sodium carbonate Na2CO3? However, an online Ideal Gas Law Calculator determines the unknown measurable properties of the ideal gas law equation. Then, molecular formula calculator Puts everything together and specify the unit of measurement as grams/mole. Now, the molecular weight calculator multiplies the atomic weight of each element by the number of atoms present in the compound. The molar mass calculator uses the chemical formula to determine the number of atoms of each element in the compound.
#MOLER MASS FINDER HOW TO#
How to Calculate Molar Mass of a Compound? When you want to determine the molecular weight of different molecules, then put these weights in the molar mass equation to find the total weight of a molecule: However, an online Chemical Equation Balancer Calculator finds a balanced equation and equilibrium constant with chemical names and formulas. Therefore, the unit of molecular weight is g/mol. In other words, the molar mass is the total mass (in grams) of all the atoms that make up one mole of a given molecule. The molecular weight of the compound determines the mass of 1 mole of the specific substance and the number of grams per mole of the compound. One mole of compound contains the Avogadro number of the molecule or formula unit (6.02214076 × 10^23 moles). It is similar to other counting units, such as a pair (2) and a dozen (12). Molar Mass Formula:Ī mole is a counting unit used to determine the number of molecules, atoms, ions, or molecular formula units in a certain compound. It can be calculated by adding the standard atomic mass of its constituent atoms. Generally, the molar mass is the ratio of the mass of a substance to the number of particles it contains. Molar mass is measured in grams per mole (g/mol). In chemistry, the molar mass of a compound is defined as the mass of the compound in the sample divided by the amount of substance in the sample. In this context, you can understand how to find molar mass and much more. In addition, the atomic mass calculator displays a pie chart for mass percentage composition by element. An online molar mass calculator allows you to calculate the molecular weight, hill notation, nominal, and monoisotopic mass for given chemical formulas.


 0 kommentar(er)
0 kommentar(er)
